Human bone marrow of healthy human donors - Annotation#
In this notebook, we annotate cyTOF data of bone marrow samples from 8 healthy donors. Data were provided by Oetjen et al (JCl Insight, 2018). We employ the following steps:
Read anndata formatted data
Annotate based on clustering
Compare to annotation provided by the authors (in publication).
import scanpy as sc
import anndata as ann
import numpy as np
import scipy as sp
import pandas as pd
import matplotlib.pyplot as pl
from matplotlib import rcParams
from matplotlib import colors
import seaborn as sb
import datetime
import pytometry as pm
sc.logging.print_versions()
sc.settings.verbosity = 3
WARNING: If you miss a compact list, please try `print_header`!
The `sinfo` package has changed name and is now called `session_info` to become more discoverable and self-explanatory. The `sinfo` PyPI package will be kept around to avoid breaking old installs and you can downgrade to 0.3.2 if you want to use it without seeing this message. For the latest features and bug fixes, please install `session_info` instead. The usage and defaults also changed slightly, so please review the latest README at https://gitlab.com/joelostblom/session_info.
-----
anndata 0.7.6
scanpy 1.8.2
sinfo 0.3.4
-----
PIL 8.4.0
anyio NA
asciitree NA
attr 21.2.0
babel 2.9.1
backcall 0.2.0
beta_ufunc NA
binom_ufunc NA
brotli 1.0.9
certifi 2021.10.08
cffi 1.15.0
charset_normalizer 2.0.7
cycler 0.10.0
cython_runtime NA
dateutil 2.8.2
debugpy 1.5.1
decorator 5.1.0
defusedxml 0.7.1
entrypoints 0.3
fasteners 0.17.3
flowio 1.0.1
google NA
h5py 2.10.0
idna 3.3
igraph 0.9.8
importlib_resources NA
ipykernel 6.5.0
ipython_genutils 0.2.0
ipywidgets 7.6.5
jedi 0.18.0
jinja2 3.0.2
joblib 1.1.0
json5 NA
jsonschema 4.2.1
jupyter_server 1.11.2
jupyterlab_server 2.8.2
kiwisolver 1.3.2
leidenalg 0.8.8
llvmlite 0.37.0
louvain 0.7.0
markupsafe 2.0.1
matplotlib 3.4.3
matplotlib_inline NA
mpl_toolkits NA
msgpack 1.0.3
natsort 8.0.0
nbclassic NA
nbformat 5.1.3
nbinom_ufunc NA
numba 0.54.1
numcodecs 0.9.1
numexpr 2.7.3
numpy 1.19.5
packaging 21.2
pandas 1.3.4
parso 0.8.2
pexpect 4.8.0
pickleshare 0.7.5
pkg_resources NA
prometheus_client NA
prompt_toolkit 3.0.22
ptyprocess 0.7.0
pvectorc NA
pydev_ipython NA
pydevconsole NA
pydevd 2.6.0
pydevd_concurrency_analyser NA
pydevd_file_utils NA
pydevd_plugins NA
pydevd_tracing NA
pygments 2.10.0
pyparsing 2.4.7
pyrsistent NA
pytometry 0.0.1
pytz 2021.3
readfcs 0.1.5
requests 2.26.0
scipy 1.7.2
seaborn 0.11.2
send2trash NA
six 1.16.0
sklearn 1.0.1
sniffio 1.2.0
socks 1.7.1
statsmodels 0.13.0
storemagic NA
tables 3.6.1
terminado 0.12.1
texttable 1.6.4
threadpoolctl 3.0.0
tornado 6.1
traitlets 5.1.1
typing_extensions NA
urllib3 1.26.7
wcwidth 0.2.5
websocket 1.2.1
yaml 6.0
zarr 2.11.3
zipp NA
zmq 22.3.0
-----
IPython 7.29.0
jupyter_client 7.0.6
jupyter_core 4.9.1
jupyterlab 3.2.2
notebook 6.4.5
-----
Python 3.8.6 (default, Oct 26 2021, 09:26:31) [GCC 8.3.0]
Linux-4.18.0-305.12.1.el8_4.x86_64-x86_64-with-glibc2.28
288 logical CPU cores
-----
Session information updated at 2022-08-10 09:26
sc.settings.figdir = "./../figures/"
Add date.
now = datetime.datetime.now()
today = now.strftime("%Y%m%d")
Define a nice colour map for marker intensity.
colors2 = pl.cm.Reds(np.linspace(0, 1, 80))
colors3 = pl.cm.Greys_r(np.linspace(0.7, 0.8, 10))
colorsComb = np.vstack([colors3, colors2])
mymap = colors.LinearSegmentedColormap.from_list("my_colormap", colorsComb)
import os
data_path = "./../data/Oetjen_2018/"
Read data#
Read the anndata object from the previous notebook. Here, we stored the arcsinh-normalised and filtered events for all donors.
adata_all = sc.read(data_path + "anndata/" + "cytof_data_norm.h5ad")
adata_all
AnnData object with n_obs × n_vars = 4829382 × 34
obs: 'sample', 'Time', 'Event-length', 'Center', 'Offset', 'Width', 'Residual', 'batch', 'DNA1', 'DNA2', 'VIABILITY'
var: 'channel', 'marker', 'signal_type', 'AB'
uns: 'meta', 'neighbors', 'pca', 'sample_colors', 'umap'
obsm: 'X_pca', 'X_umap'
varm: 'PCs'
layers: 'compensated'
obsp: 'connectivities', 'distances'
Check sample size, i.e. the number of events per donor.
adata_all.obs["sample"].value_counts()
B 940911
O 923137
H 827621
J 584961
T 503578
A 484678
C 370639
U 193857
Name: sample, dtype: int64
Exploratory data analysis#
In this section, we aim to get an overview on the data set. In particular, we check visually for batch effects (donor specific shifts in the data distribution). The PCA gives us an idea on the intrinsic dimension of the data.
sc.pl.pca_overview(adata_all, color="sample")
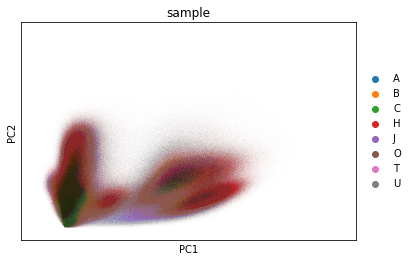
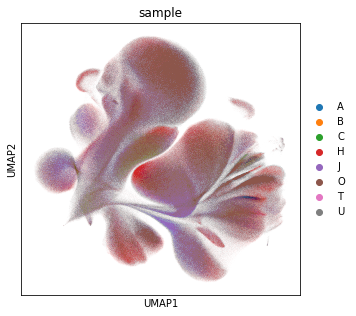
Visualise the data as UMAP (pre-computed in the previous notebook). The visual distribution of cells from different donors allows to assume that we have very little shifts due to batch effects.
rcParams["figure.figsize"] = (5, 5)
sc.pl.umap(adata_all, color="sample")
Next, we color the UMAP by all markers, where we use the custom color scheme of grey (no signal or only background) and reds to indicate the marker intensity. This map gives us an intuition for the cell distribution.
sc.pl.umap(
adata_all,
gene_symbols="AB",
color=[
"CD45",
"CD45RA",
"CD45RO",
"CD11a",
"CD16",
"CD2",
"CD5",
"CD3",
"CD4",
"CD8a",
"CD25",
"CD27",
"CD28",
"CD44",
"CD49D",
"CD57",
"CD69",
"CD7",
"CD9",
"FAS",
"HLA-DR",
"CD127",
"OX40",
"41BB",
"CTLA4",
"CD161",
"CD183",
"CD194",
"CD195",
"CD197",
"LAG3",
"ICOS",
"PD1",
"TIM3",
],
ncols=3,
color_map=mymap,
)
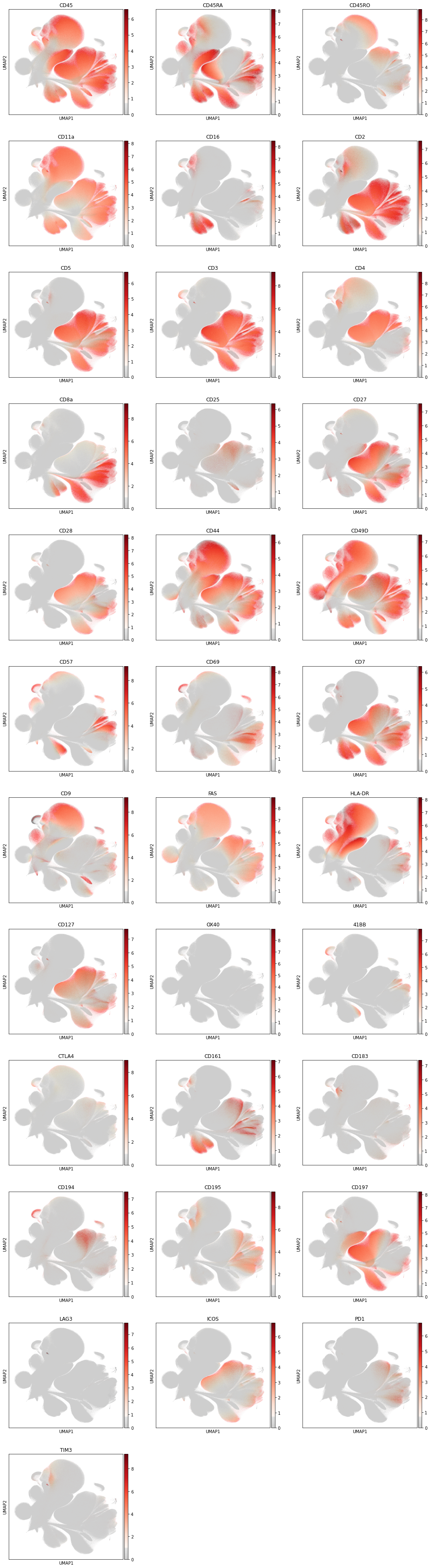
The exploratory analysis also shows that we have a variety of CD45 negative cells in the dataset. These cells are likely no immune cells. Before we continue with the cell type annotation, we filter out the CD45 negative cells.
Annotation#
For cell type annotation, we use several levels of granularity based on marker intensity. The measured panel contains mostly T cell markers. We therefore cannot annotate B cells or the myeloid lineage. In the lymphoid lineage, we can distinguish NK cells and T cells. The annotation focusses on T cell subtypes, following the annotation of the original publication. The different levels are:
Level 1: Immune cells and non-immune cells (CD45 marker)
Level 2: NK cells and T cells
Level 3: T cell subtypes (CD4, CD8, double positive and negative)
Level 4: CD4 and CD8 T cell subtypes based on marker CD197 intensity)
Level 5: naive, central and effector memory T cell subtypes
Level 1#
We start with a rough annotation into CD45+ and negative cells.
adata_all.obs["cell_type_lvl0"] = adata_all.X[:, adata_all.var["AB"] == "CD45"] > 0.5
adata_all.obs["cell_type_lvl0"] = adata_all.obs["cell_type_lvl0"].map(
{True: "CD45+", False: "CD45-"}
)
sc.pl.umap(adata_all, color="cell_type_lvl0")
/opt/python/lib/python3.8/site-packages/anndata/_core/anndata.py:1220: FutureWarning: The `inplace` parameter in pandas.Categorical.reorder_categories is deprecated and will be removed in a future version. Reordering categories will always return a new Categorical object.
c.reorder_categories(natsorted(c.categories), inplace=True)
... storing 'cell_type_lvl0' as categorical
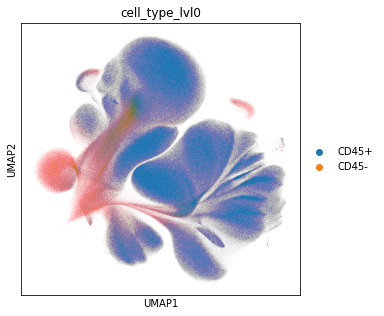
Examine the number of CD45 positive and negative cells.
adata_all.obs["cell_type_lvl0"].value_counts()
CD45+ 4162929
CD45- 666453
Name: cell_type_lvl0, dtype: int64
Level 2#
We exclude the CD45- cells and continue with annotatin the CD45+ cells.
adata_cd45 = adata_all[adata_all.obs["cell_type_lvl0"] == "CD45+"].copy()
Recompute PCA representation, UMAP embedding and clustering for subsequent cell type annotation.
sc.pp.pca(adata_cd45)
sc.pp.neighbors(adata_cd45, n_neighbors=10, n_pcs=10)
sc.tl.umap(adata_cd45)
computing PCA
with n_comps=33
finished (0:00:15)
computing neighbors
using data matrix X directly
finished: added to `.uns['neighbors']`
`.obsp['distances']`, distances for each pair of neighbors
`.obsp['connectivities']`, weighted adjacency matrix (0:17:42)
computing UMAP
sc.tl.leiden(adata_cd45, resolution=1.0)
Save temporary result to file.
adata_cd45.write(data_path + "anndata/" + "cytof_data_tmp.h5ad")
Read in the temporary data file.
adata_cd45 = sc.read(data_path + "anndata/" + "cytof_data_tmp.h5ad")
Visualize data#
Similar to the exploratory data analysis shown above, we visualise the data using UMAP. In this step, we visualise the Leiden clustering of the data. Leiden clustering is a community detection based approach that tends to overcluster the data, but captures clusters of very different sizes. In this way, we can discover very rare cells types, even though abundant cell types are split into several subclusters.
rcParams["figure.figsize"] = (5, 5)
sc.pl.umap(adata_cd45, color="leiden")
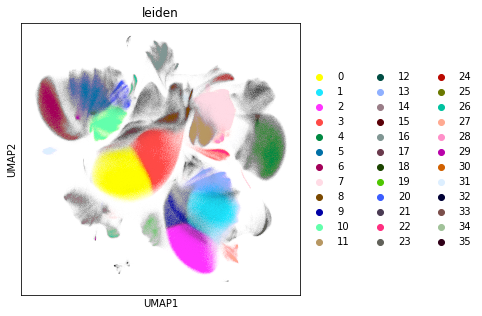
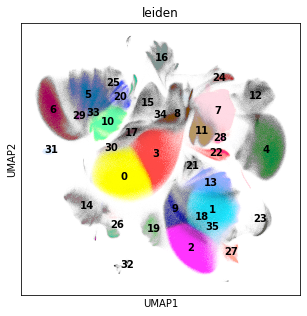
rcParams["figure.figsize"] = (5, 5)
sc.pl.umap(adata_cd45, color="leiden", legend_loc="on data")
Let us inspect the marker intensity on a UMAP.
sc.pl.umap(
adata_cd45,
gene_symbols="AB",
color=[
"CD45",
"CD45RA",
"CD45RO",
"CD11a",
"CD16",
"CD2",
"CD5",
"CD3",
"CD4",
"CD8a",
"CD25",
"CD27",
"CD28",
"CD44",
"CD49D",
"CD57",
"CD69",
"CD7",
"CD9",
"CD95-FAS",
"HLA-DR",
"CD127",
"CD134-OX40",
"CD137-41BB",
"CD152-CTLA4",
"CD161",
"CD183",
"CD194",
"CD195",
"CD197",
"CD223-LAG3",
"CD278-ICOS",
"CD279-PD1",
"CD366-TIM3",
],
ncols=3,
color_map=mymap,
vmax="p99",
)
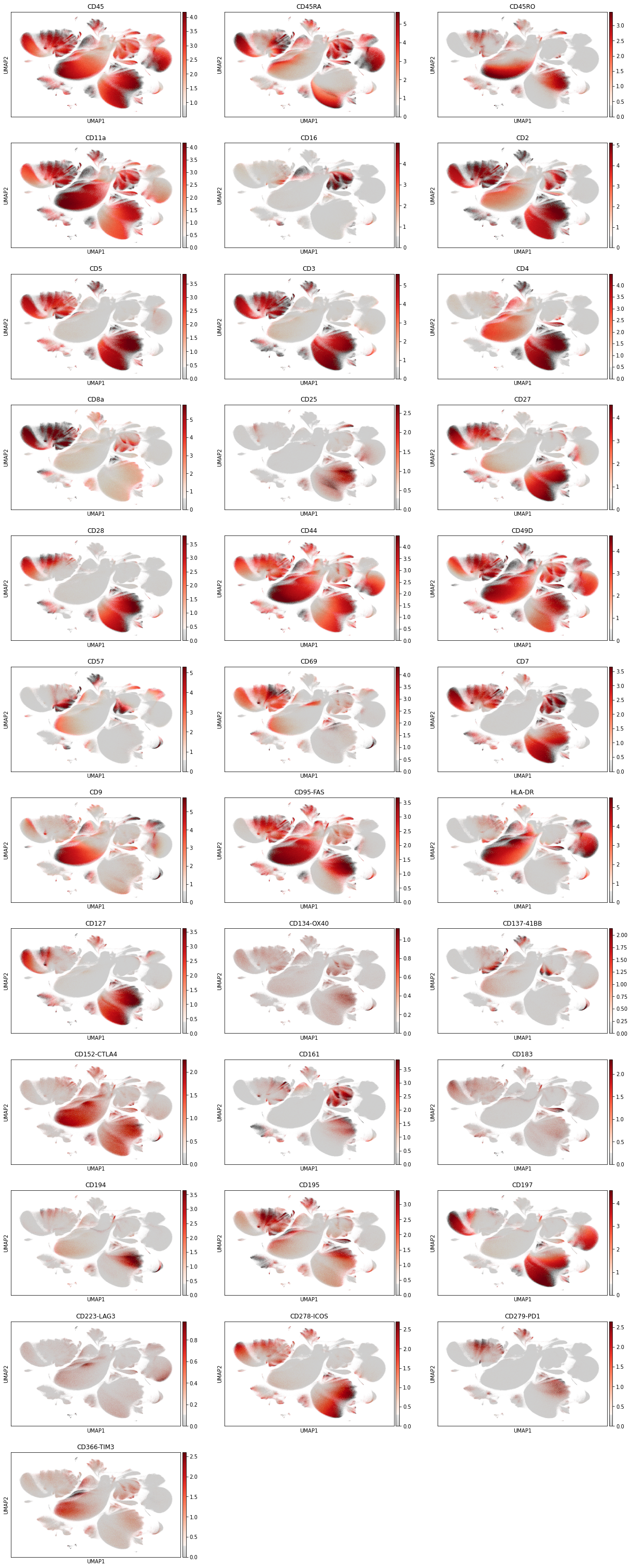
Let us inspect the mean marker intensity as a matrixplot. Clusters are organised based on hierarchical clustering.
sc.pl.matrixplot(
adata_cd45,
groupby="leiden",
gene_symbols="AB",
var_names=[
"CD45",
"CD45RA",
"CD45RO",
"CD11a",
"CD16",
"CD2",
"CD5",
"CD3",
"CD4",
"CD8a",
"CD25",
"CD27",
"CD28",
"CD44",
"CD49D",
"CD57",
"CD69",
"CD7",
"CD9",
"CD95-FAS",
"HLA-DR",
"CD127",
"CD134-OX40",
"CD137-41BB",
"CD152-CTLA4",
"CD161",
"CD183",
"CD194",
"CD195",
"CD197",
"CD223-LAG3",
"CD278-ICOS",
"CD279-PD1",
"CD366-TIM3",
],
dendrogram=True,
vmin=0,
cmap=mymap,
)
WARNING: dendrogram data not found (using key=dendrogram_leiden). Running `sc.tl.dendrogram` with default parameters. For fine tuning it is recommended to run `sc.tl.dendrogram` independently.
using data matrix X directly
Storing dendrogram info using `.uns['dendrogram_leiden']`
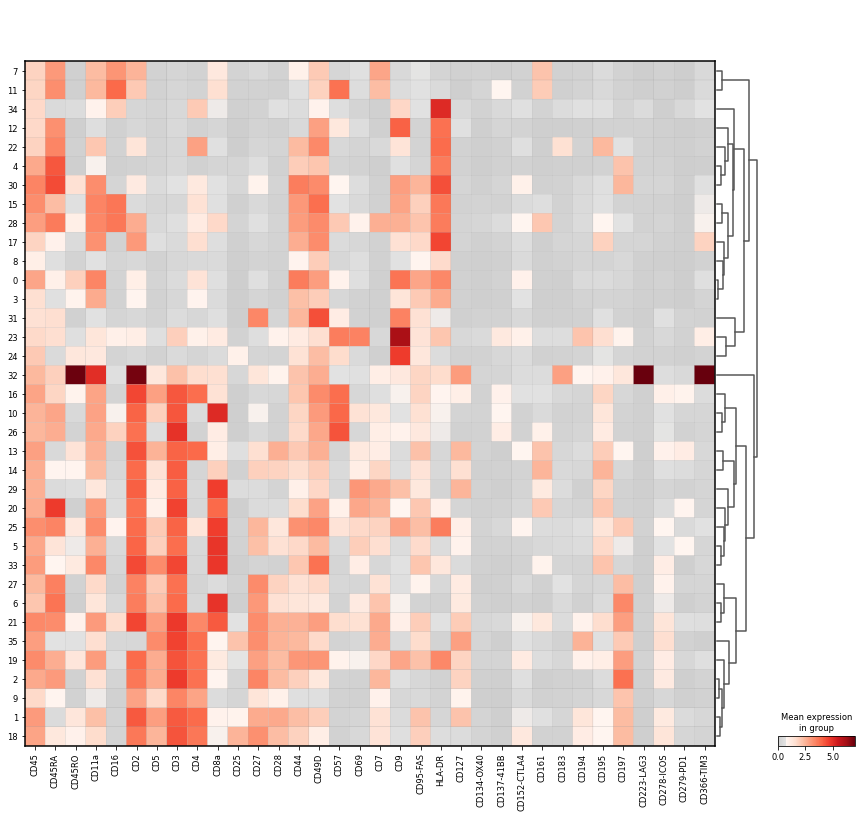
Markers for NK cells:
CD16+
HLA-DR-
CD3-
CD44-
CD45RA+
Markers for neutrophils (in distinction to NK cells):
CD16+
CD44+
CD3-
Markers for T cells:
CD3+ (general marker for T cells)
CD4+
CD8a+
Annotate T cells and NK cells in a second round.
cluster2cell = {
"0": "not annotated",
"1": "T cell", #
"2": "T cell", #
"3": "not annotated",
"4": "not annotated",
"5": "T cell", #
"6": "T cell", #
"7": "NK cell", #
"8": "not annotated",
"9": "T cell", #
"10": "T cell", #
"11": "NK cell", #
"12": "not annotated",
"13": "T cell", #
"14": "T cell", #
"15": "not annotated",
"16": "T cell", #
"17": "not annotated",
"18": "T cell", #
"19": "T cell", #
"20": "T cell", #
"21": "T cell", # Double positive CD4/CD8
"22": "not annotated",
"23": "not annotated",
"24": "not annotated",
"25": "T cell", #
"26": "T cell", #
"27": "T cell", #
"28": "NK cell", # HLA-DR positive NK cell?
"29": "T cell", #
"30": "not annotated",
"31": "not annotated",
"32": "not annotated",
"33": "T cell", #
"34": "not annotated",
"35": "T cell", #
}
adata_cd45.obs["cell_type_lvl2"] = adata_cd45.obs["leiden"].map(cluster2cell).copy()
sc.pl.umap(adata_cd45, color="cell_type_lvl2")
/opt/python/lib/python3.8/site-packages/anndata/_core/anndata.py:1220: FutureWarning: The `inplace` parameter in pandas.Categorical.reorder_categories is deprecated and will be removed in a future version. Reordering categories will always return a new Categorical object.
c.reorder_categories(natsorted(c.categories), inplace=True)
... storing 'cell_type_lvl2' as categorical
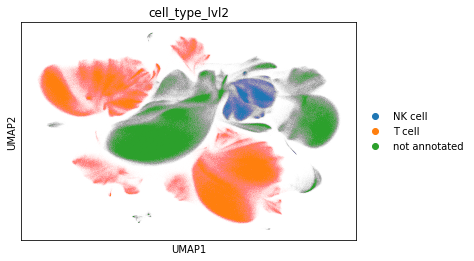
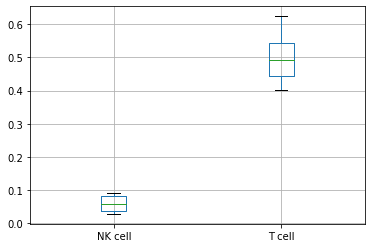
df = pd.crosstab(
adata_cd45.obs["sample"], adata_cd45.obs["cell_type_lvl2"], normalize=0
)
df
| cell_type_lvl2 | NK cell | T cell | not annotated |
|---|---|---|---|
| sample | |||
| A | 0.048344 | 0.624290 | 0.327366 |
| B | 0.092156 | 0.472933 | 0.434912 |
| C | 0.031945 | 0.403321 | 0.564733 |
| H | 0.084707 | 0.571623 | 0.343670 |
| J | 0.028097 | 0.510479 | 0.461425 |
| O | 0.069115 | 0.448752 | 0.482133 |
| T | 0.083106 | 0.431413 | 0.485481 |
| U | 0.037466 | 0.533421 | 0.429112 |
ax = sb.boxplot(data=df[["NK cell", "T cell"]], orient="v")
ax = sb.swarmplot(
data=df[["NK cell", "T cell"]], orient="v", color=".25", size=10, alpha=0.8
)
ax.set_ylim([0, 1])
(0.0, 1.0)
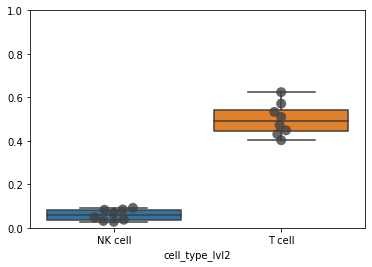
boxplot = df.boxplot(column=["NK cell", "T cell"])
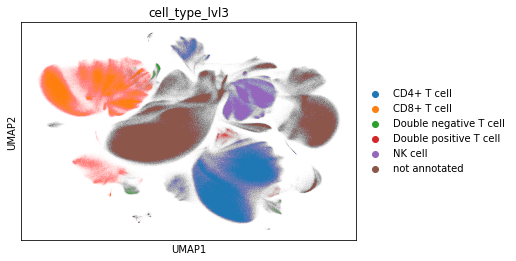
Level 3#
Annotate T cell subtypes in a third round. Notes on markers:
Double positive T cells: express both CD4 and CD8a
Double negative T cells: express only CD3, but not CD4 or CD8a
Distinguish CD4+ T cells and CD8+ T cells
CCR7 is also known as CD197
cluster2cell = {
"0": "not annotated",
"1": "CD4+ T cell", #
"2": "CD4+ T cell", #
"3": "not annotated",
"4": "not annotated",
"5": "CD8+ T cell", #
"6": "CD8+ T cell", #
"7": "NK cell", #
"8": "not annotated",
"9": "CD4+ T cell", #
"10": "CD8+ T cell", #
"11": "NK cell", #
"12": "not annotated",
"13": "CD4+ T cell", #
"14": "CD8+ T cell", #
"15": "not annotated",
"16": "CD4+ T cell", #
"17": "not annotated",
"18": "CD4+ T cell", #
"19": "CD4+ T cell", #
"20": "CD8+ T cell", #
"21": "Double positive T cell", # Double positive CD4/CD8
"22": "not annotated",
"23": "not annotated",
"24": "not annotated",
"25": "CD8+ T cell", #
"26": "Double negative T cell", # very little CD8a
"27": "Double negative T cell", # has only CD3 marker
"28": "NK cell", # HLA-DR positive NK cell?
"29": "CD8+ T cell", #
"30": "not annotated",
"31": "not annotated",
"32": "not annotated", # special activated T cell or simply autofluorescence?
"33": "CD8+ T cell", #
"34": "not annotated",
"35": "CD4+ T cell", #
}
adata_cd45.obs["cell_type_lvl3"] = adata_cd45.obs["leiden"].map(cluster2cell).copy()
sc.pl.umap(adata_cd45, color="cell_type_lvl3")
/opt/python/lib/python3.8/site-packages/anndata/_core/anndata.py:1220: FutureWarning: The `inplace` parameter in pandas.Categorical.reorder_categories is deprecated and will be removed in a future version. Reordering categories will always return a new Categorical object.
c.reorder_categories(natsorted(c.categories), inplace=True)
... storing 'cell_type_lvl3' as categorical
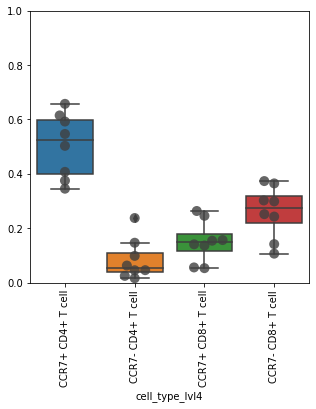
Check proportions. Restrict to CD4 and CD8 T cells.
obs_tmp = adata_cd45.obs.loc[
adata_cd45.obs["cell_type_lvl3"].isin(["CD4+ T cell", "CD8+ T cell"])
]
df = pd.crosstab(obs_tmp["sample"], obs_tmp["cell_type_lvl4"], normalize=0)
df
| cell_type_lvl4 | CCR7+ CD4+ T cell | CCR7+ CD8+ T cell | CCR7- CD4+ T cell | CCR7- CD8+ T cell |
|---|---|---|---|---|
| sample | ||||
| A | 0.345225 | 0.052842 | 0.237266 | 0.364666 |
| B | 0.592302 | 0.140645 | 0.024481 | 0.242573 |
| C | 0.546661 | 0.055512 | 0.146351 | 0.251477 |
| H | 0.502886 | 0.136178 | 0.062442 | 0.298493 |
| J | 0.614723 | 0.263139 | 0.015905 | 0.106233 |
| O | 0.374888 | 0.153879 | 0.097987 | 0.373247 |
| T | 0.407082 | 0.245514 | 0.045433 | 0.301972 |
| U | 0.657344 | 0.156213 | 0.045153 | 0.141291 |
ax = sb.boxplot(
data=df[
[
"CCR7+ CD4+ T cell",
"CCR7- CD4+ T cell",
"CCR7+ CD8+ T cell",
"CCR7- CD8+ T cell",
]
],
orient="v",
)
ax = sb.swarmplot(
data=df[
[
"CCR7+ CD4+ T cell",
"CCR7- CD4+ T cell",
"CCR7+ CD8+ T cell",
"CCR7- CD8+ T cell",
]
],
orient="v",
color=".25",
size=10,
alpha=0.8,
)
ax.set_xticklabels(ax.get_xticklabels(), rotation=90)
ax.set_ylim([0, 1])
(0.0, 1.0)
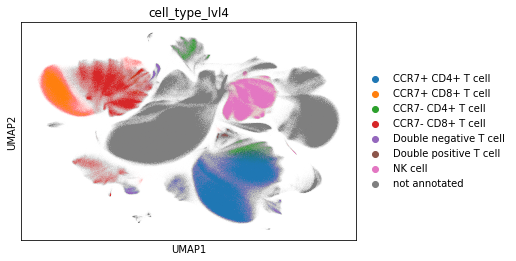
Level 4#
Annotate T cell subtypes in a fourth round. Notes on markers:
Distinguish CCR7+/- cells in CD4+ T cells and CD8+ T cells
CCR7 is also known as CD197
cluster2cell = {
"0": "not annotated",
"1": "CCR7+ CD4+ T cell", #
"2": "CCR7+ CD4+ T cell", #
"3": "not annotated",
"4": "not annotated",
"5": "CCR7- CD8+ T cell", #
"6": "CCR7+ CD8+ T cell", #
"7": "NK cell", #
"8": "not annotated",
"9": "CCR7+ CD4+ T cell", #
"10": "CCR7- CD8+ T cell", #
"11": "NK cell", #
"12": "not annotated",
"13": "CCR7- CD4+ T cell", #
"14": "CCR7- CD8+ T cell", #
"15": "not annotated",
"16": "CCR7- CD4+ T cell", #
"17": "not annotated",
"18": "CCR7+ CD4+ T cell", #
"19": "CCR7+ CD4+ T cell", #
"20": "CCR7- CD8+ T cell", #
"21": "Double positive T cell", # Double positive CD4/CD8
"22": "not annotated",
"23": "not annotated",
"24": "not annotated",
"25": "CCR7+ CD8+ T cell", #
"26": "Double negative T cell", # very little CD8a
"27": "Double negative T cell", # has only CD3 marker
"28": "NK cell", # HLA-DR positive NK cell?
"29": "CCR7- CD8+ T cell", #
"30": "not annotated",
"31": "not annotated",
"32": "not annotated", # special activated T cell or simply autofluorescence?
"33": "CCR7- CD8+ T cell", #
"34": "not annotated",
"35": "CCR7+ CD4+ T cell", #
}
adata_cd45.obs["cell_type_lvl4"] = adata_cd45.obs["leiden"].map(cluster2cell).copy()
sc.pl.umap(adata_cd45, color="cell_type_lvl4")
/opt/python/lib/python3.8/site-packages/anndata/_core/anndata.py:1220: FutureWarning: The `inplace` parameter in pandas.Categorical.reorder_categories is deprecated and will be removed in a future version. Reordering categories will always return a new Categorical object.
c.reorder_categories(natsorted(c.categories), inplace=True)
... storing 'cell_type_lvl4' as categorical
Check proportions. Restrict to CD4 and CD8 T cells.
obs_tmp = adata_cd45.obs.loc[
adata_cd45.obs["cell_type_lvl3"].isin(["CD4+ T cell", "CD8+ T cell"])
]
df = pd.crosstab(obs_tmp["sample"], obs_tmp["cell_type_lvl4"], normalize=0)
df
| cell_type_lvl4 | CCR7+ CD4+ T cell | CCR7+ CD8+ T cell | CCR7- CD4+ T cell | CCR7- CD8+ T cell |
|---|---|---|---|---|
| sample | ||||
| A | 0.345225 | 0.052842 | 0.237266 | 0.364666 |
| B | 0.592302 | 0.140645 | 0.024481 | 0.242573 |
| C | 0.546661 | 0.055512 | 0.146351 | 0.251477 |
| H | 0.502886 | 0.136178 | 0.062442 | 0.298493 |
| J | 0.614723 | 0.263139 | 0.015905 | 0.106233 |
| O | 0.374888 | 0.153879 | 0.097987 | 0.373247 |
| T | 0.407082 | 0.245514 | 0.045433 | 0.301972 |
| U | 0.657344 | 0.156213 | 0.045153 | 0.141291 |
ax = sb.boxplot(
data=df[
[
"CCR7+ CD4+ T cell",
"CCR7- CD4+ T cell",
"CCR7+ CD8+ T cell",
"CCR7- CD8+ T cell",
]
],
orient="v",
)
ax = sb.swarmplot(
data=df[
[
"CCR7+ CD4+ T cell",
"CCR7- CD4+ T cell",
"CCR7+ CD8+ T cell",
"CCR7- CD8+ T cell",
]
],
orient="v",
color=".25",
size=10,
alpha=0.8,
)
ax.set_xticklabels(ax.get_xticklabels(), rotation=90)
ax.set_ylim([0, 1])
(0.0, 1.0)

Level 5#
Annotate T cell subtypes in a fifth round. Notes on markers:
Distinguish Naive, Central Memory (CM), Effector Memory (EM) and terminally differentiated effector memory T cells (TEMRA) in CD4+ T cells and CD8+ T cells with CD45RA:
CCR7+ CD45RA+ is a Naive T cell
CCR7+ CD45RA- is a CM T cell
CCR7- CD45RA+ is a TEMRA
CCR7- CD45RA+ in CD8+ T cells is an effector T cell (TE)
CCR7- CD45RA- is an EM T cell
CCR7- CD45RA- CD69+ is a Tissue-resident T cell (TRM)
CCR7 is also known as CD197
cluster2cell = {
"0": "not annotated",
"1": "CD4+ CM T cell", #
"2": "Naive CD4+ T cell", #
"3": "not annotated",
"4": "not annotated",
"5": "CD8+ EM T cell", #
"6": "Naive CD8+ T cell", #
"7": "NK cell", #
"8": "not annotated",
"9": "CD4+ CM T cell", #
"10": "CD8+ TE T cell", #
"11": "NK cell", #
"12": "not annotated",
"13": "CD4+ EM T cell", # mixed with TRM CCR7- CD45RA- CD69+
"14": "CD8+ EM T cell", # somewhat different to cluster 5
"15": "not annotated",
"16": "CD4+ TEMRA", #
"17": "not annotated",
"18": "CD4+ CM T cell", #
"19": "Naive CD4+ T cell", #
"20": "CD8+ TE T cell", #
"21": "Double positive T cell", # Double positive CD4/CD8
"22": "not annotated",
"23": "not annotated",
"24": "not annotated",
"25": "Naive CD8+ T cell", #
"26": "Double negative T cell", # very little CD8a
"27": "Double negative T cell", # has only CD3 marker
"28": "NK cell", # HLA-DR positive NK cell?
"29": "CD8+ TRM T cell", # CCR7- CD45RA- CD69+
"30": "not annotated",
"31": "not annotated",
"32": "not annotated", # special activated T cell or simply autofluorescence?
"33": "CD8+ EM T cell", #
"34": "not annotated",
"35": "CD4+ CM T cell", #
}
adata_cd45.obs["cell_type_lvl5"] = pd.Categorical(
adata_cd45.obs["leiden"].map(cluster2cell).copy()
)
Subcluster#
In addition to the initial clustering, we observe that cluster 13 is a mixture of CD4+ TRM cells and CD4+ EM T cells. Let us subcluster cluster 13 to resolve CD4+ TRMs.
sc.tl.leiden(adata_cd45, key_added="leiden_R", restrict_to=["leiden", ["13"]])
running Leiden clustering
finished: found 52 clusters and added
'leiden_R', the cluster labels (adata.obs, categorical) (0:00:46)
Cluster 5 is a mixture of naive CD8+ cells and CD8+ CM T cells. Subcluster cluster 5 to resolve CD8+ CMs.
sc.tl.leiden(adata_cd45, key_added="leiden_R", restrict_to=["leiden_R", ["5"]])
running Leiden clustering
finished: found 67 clusters and added
'leiden_R', the cluster labels (adata.obs, categorical) (0:07:12)
rcParams["figure.figsize"] = (10, 10)
sc.pl.umap(adata_cd45, color="leiden_R", legend_loc="on data")
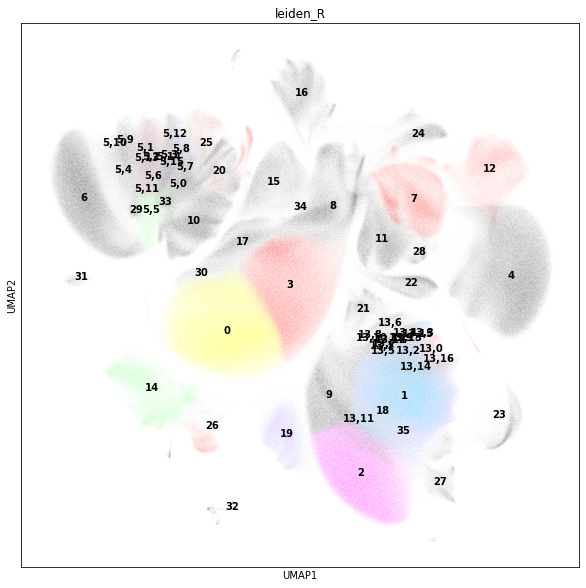
Let us visualise the mean marker intensity as matrixplot to examine the intensity levels in the subclustered data. Also, we want to see where the subclusters are grouped.
sc.pl.matrixplot(
adata_cd45,
groupby="leiden_R",
gene_symbols="AB",
var_names=[
"CD45",
"CD45RA",
"CD45RO",
"CD11a",
"CD16",
"CD2",
"CD5",
"CD3",
"CD4",
"CD8a",
"CD197",
"CD25",
"CD27",
"CD28",
"CD44",
"CD49D",
"CD57",
"CD69",
"CD7",
"CD9",
"CD95-FAS",
"HLA-DR",
"CD127",
"CD134-OX40",
"CD137-41BB",
"CD152-CTLA4",
"CD161",
"CD183",
"CD194",
"CD195",
"CD223-LAG3",
"CD278-ICOS",
"CD279-PD1",
"CD366-TIM3",
],
dendrogram=True,
vmin=0,
cmap=mymap,
)
WARNING: dendrogram data not found (using key=dendrogram_leiden_R). Running `sc.tl.dendrogram` with default parameters. For fine tuning it is recommended to run `sc.tl.dendrogram` independently.
using data matrix X directly
Storing dendrogram info using `.uns['dendrogram_leiden_R']`
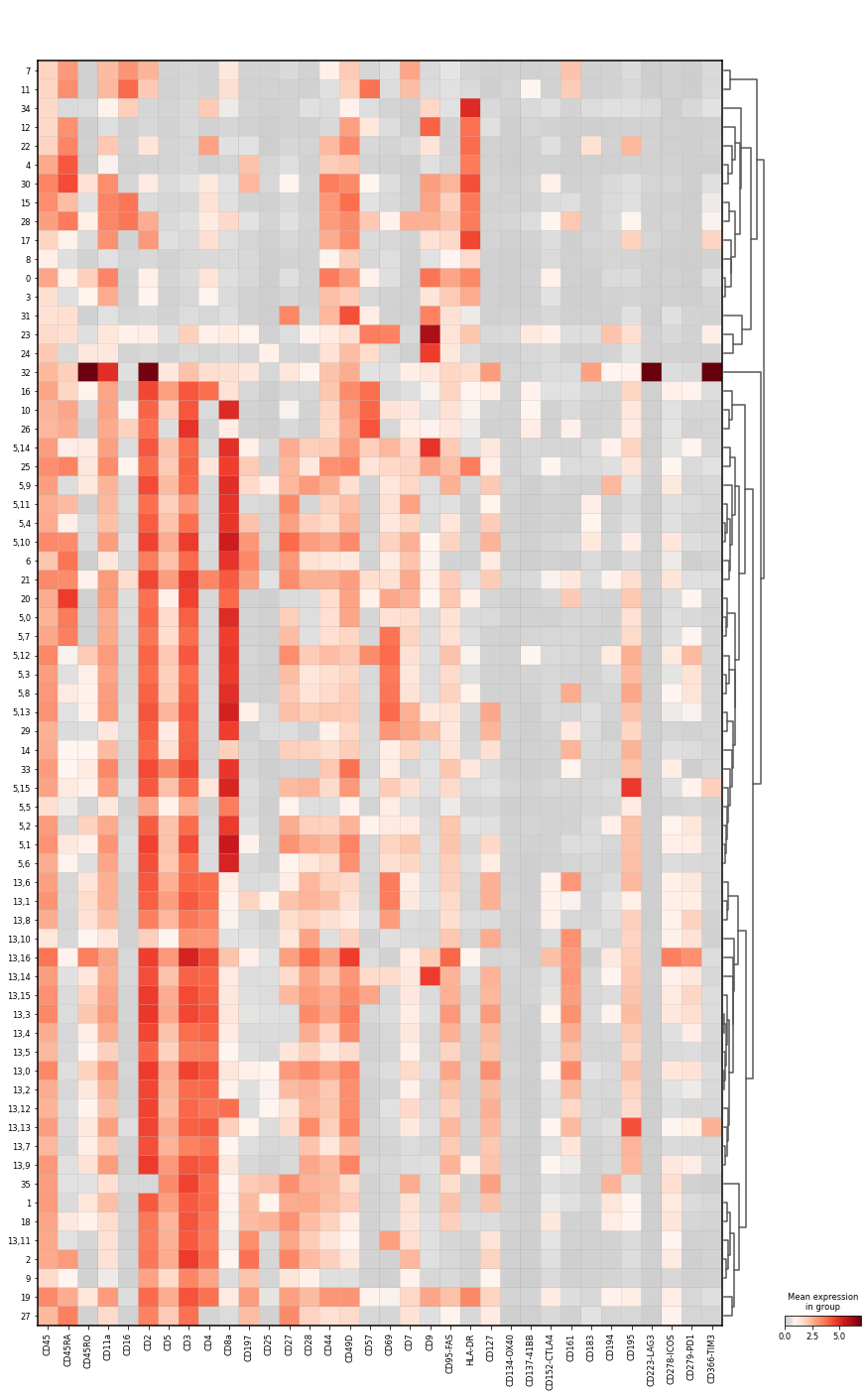
Annotate cells from subclustering#
Clusters 13,6 and 13,8 CD4+ T cells, which are negative for CD197 and CD45RA, so we term them CD4+ TRM T cell. In contrast, 13,1 is slightly positive for CD197, so we keep the original annotation. All three clusters have a distinctly higher intensity for CD69, though.
adata_cd45.obs["cell_type_lvl5"] = adata_cd45.obs["cell_type_lvl5"].cat.add_categories(
["CD4+ TRM T cell"]
)
adata_cd45.obs["cell_type_lvl5"][
adata_cd45.obs["leiden_R"].isin(["13,6", "13,8"])
] = "CD4+ TRM T cell"
Clusters 5,4 and 5,9 are CD8+ T cells, which are positive for CD197 and negative for CD45RA, so we term them CD8+ CM T cells. Cluster 5,10 is double positive for CD197 and CD45RA, which is characteristic for naive CD8+ T cells.
adata_cd45.obs["cell_type_lvl5"] = adata_cd45.obs["cell_type_lvl5"].cat.add_categories(
["CD8+ CM T cell"]
)
adata_cd45.obs["cell_type_lvl5"][
adata_cd45.obs["leiden_R"].isin(["5,4", "5,9"])
] = "CD8+ CM T cell"
adata_cd45.obs["cell_type_lvl5"][
adata_cd45.obs["leiden_R"].isin(["5,10"])
] = "Naive CD8+ T cell"
adata_cd45.obs["cell_type_lvl5"] = adata_cd45.obs[
"cell_type_lvl5"
].cat.remove_unused_categories()
Save to file.
adata_cd45.write(data_path + "anndata/" + "cytof_data_annotated.h5ad")
Visualise final annotation#
Read anndata object with final annotation.
adata_cd45 = sc.read(data_path + "anndata/" + "cytof_data_annotated.h5ad")
Reorder the cell type names for visualisation.
adata_cd45.obs["cell_type_lvl5"] = adata_cd45.obs[
"cell_type_lvl5"
].cat.reorder_categories(
[
"Naive CD4+ T cell",
"CD4+ CM T cell",
"CD4+ EM T cell",
"CD4+ TRM T cell",
"CD4+ TEMRA",
"Naive CD8+ T cell",
"CD8+ CM T cell",
"CD8+ EM T cell",
"CD8+ TRM T cell",
"CD8+ TE T cell",
"NK cell",
"Double negative T cell",
"Double positive T cell",
"not annotated",
]
)
Adjust color scheme and set gray as color for not annotated cells.
adata_cd45.uns["cell_type_lvl5_colors"][:-1] = np.flip(
adata_cd45.uns["cell_type_lvl5_colors"][:-1]
)
adata_cd45.uns["cell_type_lvl5_colors"][-1] = "#bbbbbb" # not annotated
Plot the mean marker intensity for all cell types in the highest level of granularity. Save the plots as PDF and PNG file.
sc.pl.matrixplot(
adata_cd45,
groupby="cell_type_lvl5",
gene_symbols="AB",
var_names=[
"CD45",
"HLA-DR",
"CD16",
"CD44",
"CD3",
"CD4",
"CD8a",
"CD197",
"CD45RA",
"CD57",
"CD69",
"CD2",
"CD5",
"CD7",
"CD9",
"CD11a",
"CD25",
"CD27",
"CD28",
"CD45RO",
"CD49D",
"CD95-FAS",
"CD127",
"CD134-OX40",
"CD137-41BB",
"CD152-CTLA4",
"CD161",
"CD183",
"CD194",
"CD195",
"CD223-LAG3",
"CD278-ICOS",
"CD279-PD1",
"CD366-TIM3",
],
dendrogram=False,
vmin=0, # standard_scale='var',
save=f"{today}_Tcell_subtypes.pdf",
cmap=mymap,
)
WARNING: saving figure to file ../figures/matrixplot_20220728_Tcell_subtypes.pdf
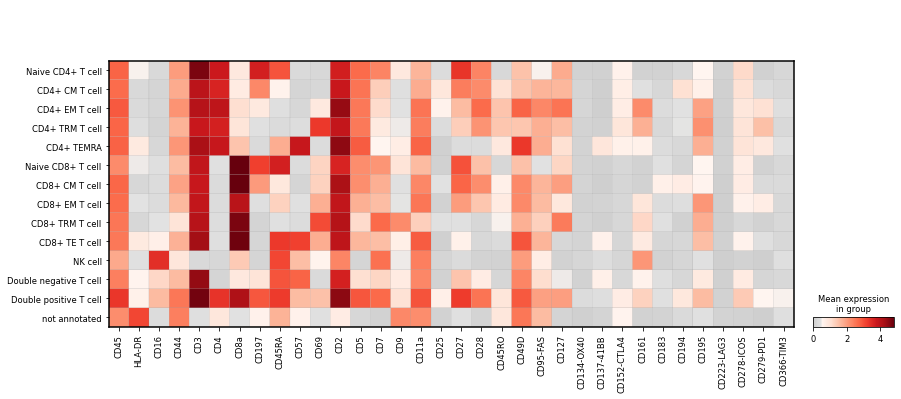
sc.pl.matrixplot(
adata_cd45,
groupby="cell_type_lvl5",
gene_symbols="AB",
var_names=[
"CD45",
"HLA-DR",
"CD16",
"CD44",
"CD3",
"CD4",
"CD8a",
"CD197",
"CD45RA",
"CD57",
"CD69",
"CD2",
"CD5",
"CD7",
"CD9",
"CD11a",
"CD25",
"CD27",
"CD28",
"CD45RO",
"CD49D",
"CD95-FAS",
"CD127",
"CD134-OX40",
"CD137-41BB",
"CD152-CTLA4",
"CD161",
"CD183",
"CD194",
"CD195",
"CD223-LAG3",
"CD278-ICOS",
"CD279-PD1",
"CD366-TIM3",
],
dendrogram=False,
vmin=0, # standard_scale='var',
save=f"{today}_Tcell_subtypes.png",
cmap=mymap,
)
WARNING: saving figure to file ../figures/matrixplot_20220728_Tcell_subtypes.png

Visualise the cell type annotation on a UMAP plot. Save the plot as PDF and PNG file.
rcParams["figure.figsize"] = (5, 5)
sc.pl.umap(
adata_cd45, color="cell_type_lvl5", save="_" + today + "_cytof_cd45_lvl5.pdf"
)
WARNING: saving figure to file ../figures/umap_20220323_cytof_cd45_lvl5.pdf
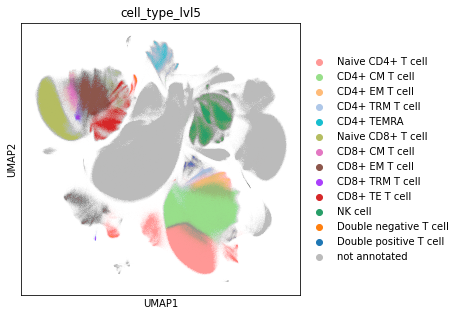
rcParams["figure.figsize"] = (5, 5)
sc.pl.umap(
adata_cd45, color="cell_type_lvl5", save="_" + today + "_cytof_cd45_lvl5.png"
)
WARNING: saving figure to file ../figures/umap_20220323_cytof_cd45_lvl5.png

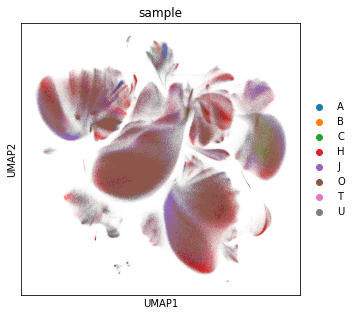
Visualise the cells colored by donor on a UMAP and save as PDF and PNG file.
rcParams["figure.figsize"] = (5, 5)
sc.pl.umap(adata_cd45, color="sample", save="_" + today + "_cytof_donor.pdf")
WARNING: saving figure to file ../figures/umap_20220728_cytof_donor.pdf
rcParams["figure.figsize"] = (5, 5)
sc.pl.umap(adata_cd45, color="sample", save="_" + today + "_cytof_donor.png")
WARNING: saving figure to file ../figures/umap_20220728_cytof_donor.png

Boxplot of cell fractions#
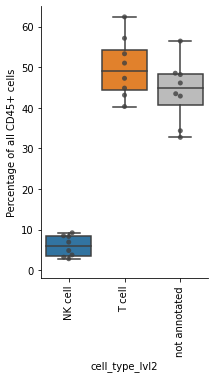
To check our annotation, we compute the proportions of all immune cell types.
obs_tmp = adata_cd45.obs
df = pd.crosstab(obs_tmp["sample"], obs_tmp["cell_type_lvl2"], normalize=0) * 100
df
| cell_type_lvl2 | NK cell | T cell | not annotated |
|---|---|---|---|
| sample | |||
| A | 4.834401 | 62.429026 | 32.736573 |
| B | 9.215583 | 47.293266 | 43.491151 |
| C | 3.194541 | 40.332118 | 56.473341 |
| H | 8.470708 | 57.162303 | 34.366989 |
| J | 2.809668 | 51.047882 | 46.142450 |
| O | 6.911501 | 44.875164 | 48.213335 |
| T | 8.310561 | 43.141323 | 48.548116 |
| U | 3.746645 | 53.342113 | 42.911242 |
df.columns
CategoricalIndex(['NK cell', 'T cell', 'not annotated'], categories=['NK cell', 'T cell', 'not annotated'], ordered=False, dtype='category', name='cell_type_lvl2')
Show the proportions of NK cells, T cells and the not annotated cell types from the lowest level of granularity. Save the plot as PDF and PNG file.
rcParams["figure.figsize"] = (3, 5)
ax = sb.boxplot(
data=df[["NK cell", "T cell", "not annotated"]],
orient="v",
palette=["#1f77b4", "#ff7f0e", "#bbbbbb"],
)
ax = sb.swarmplot(
data=df[["NK cell", "T cell", "not annotated"]],
orient="v",
color=".25",
size=5,
alpha=0.8,
)
ax.set_xticklabels(ax.get_xticklabels(), rotation=90)
ax.spines["right"].set_visible(False)
ax.spines["top"].set_visible(False)
ax.set_ylim([-2, 65])
ax.set_ylabel("Percentage of all CD45+ cells")
pl.savefig(f"./../figures/{today}_boxplot_Oetjen_cytof_CD45.pdf", bbox_inches="tight")
pl.show()
rcParams["figure.figsize"] = (3, 5)
ax = sb.boxplot(
data=df[["NK cell", "T cell", "not annotated"]],
orient="v",
palette=["#1f77b4", "#ff7f0e", "#bbbbbb"],
)
ax = sb.swarmplot(
data=df[["NK cell", "T cell", "not annotated"]],
orient="v",
color=".25",
size=5,
alpha=0.8,
)
ax.set_xticklabels(ax.get_xticklabels(), rotation=90)
ax.spines["right"].set_visible(False)
ax.spines["top"].set_visible(False)
ax.set_ylim([-2, 65])
ax.set_ylabel("Percentage of all CD45+ cells")
pl.savefig(f"./../figures/{today}_boxplot_Oetjen_cytof_CD45.png", bbox_inches="tight")
pl.show()

Boxplot of T cell fractions#
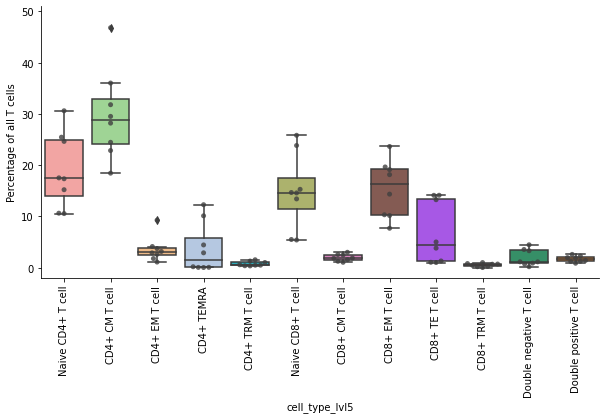
Next, the compute the proportions of T cell subtypes. Here we exclude the NK cells and not annotated cells.
obs_tmp = adata_cd45.obs.loc[
adata_cd45.obs["cell_type_lvl3"].isin(
[ #'NK cell',
"CD4+ T cell",
"CD8+ T cell",
"Double negative T cell",
"Double positive T cell",
]
)
]
df = pd.crosstab(obs_tmp["sample"], obs_tmp["cell_type_lvl5"], normalize=0) * 100
df
| cell_type_lvl5 | Naive CD4+ T cell | CD4+ CM T cell | CD4+ EM T cell | CD4+ TRM T cell | CD4+ TEMRA | Naive CD8+ T cell | CD8+ CM T cell | CD8+ EM T cell | CD8+ TRM T cell | CD8+ TE T cell | Double negative T cell | Double positive T cell |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sample | ||||||||||||
| A | 15.218739 | 18.446668 | 9.277919 | 1.562233 | 12.297399 | 5.413229 | 1.259448 | 19.655145 | 0.260562 | 14.125899 | 0.203885 | 2.278874 |
| B | 25.461977 | 31.797843 | 1.793686 | 0.501674 | 0.071262 | 14.594000 | 3.018309 | 14.359816 | 0.045442 | 5.029396 | 0.719333 | 2.607261 |
| C | 17.528144 | 36.018856 | 3.178619 | 1.026850 | 10.130046 | 5.510870 | 1.849318 | 18.156945 | 0.724399 | 3.828847 | 1.161181 | 0.885926 |
| H | 24.642163 | 22.860104 | 2.627395 | 0.357778 | 2.913099 | 13.428097 | 2.484190 | 10.187133 | 0.819662 | 14.139649 | 3.561057 | 1.979673 |
| J | 30.615129 | 29.517360 | 1.112910 | 0.390318 | 0.052575 | 25.830372 | 1.065803 | 7.704990 | 0.206515 | 1.324473 | 0.901769 | 1.277786 |
| O | 10.641064 | 24.461048 | 4.141803 | 0.583726 | 4.449321 | 14.672224 | 1.583755 | 19.141179 | 0.690654 | 13.268893 | 4.477711 | 1.888620 |
| T | 10.567680 | 28.205243 | 3.732956 | 0.509420 | 0.084903 | 23.841655 | 2.576148 | 23.647271 | 1.011579 | 1.069112 | 3.319611 | 1.434421 |
| U | 17.355147 | 46.807631 | 2.898825 | 1.308842 | 0.199634 | 15.293840 | 1.898293 | 10.336070 | 0.485500 | 1.025338 | 1.152915 | 1.237966 |
df.columns
CategoricalIndex(['Naive CD4+ T cell', 'CD4+ CM T cell', 'CD4+ EM T cell',
'CD4+ TRM T cell', 'CD4+ TEMRA', 'Naive CD8+ T cell',
'CD8+ CM T cell', 'CD8+ EM T cell', 'CD8+ TRM T cell',
'CD8+ TE T cell', 'Double negative T cell',
'Double positive T cell'],
categories=['Naive CD4+ T cell', 'CD4+ CM T cell', 'CD4+ EM T cell', 'CD4+ TRM T cell', 'CD4+ TEMRA', 'Naive CD8+ T cell', 'CD8+ CM T cell', 'CD8+ EM T cell', ...], ordered=False, dtype='category', name='cell_type_lvl5')
Plot the proportions of T cell subtypes as boxplots. Every dot is the proportion from a single donor. Save the plots as PDF and PNG file.
rcParams["figure.figsize"] = (10, 5)
ax = sb.boxplot(
data=df[
[
"Naive CD4+ T cell",
"CD4+ CM T cell",
"CD4+ EM T cell",
"CD4+ TEMRA",
"CD4+ TRM T cell",
"Naive CD8+ T cell",
"CD8+ CM T cell",
"CD8+ EM T cell",
"CD8+ TE T cell",
"CD8+ TRM T cell",
"Double negative T cell",
"Double positive T cell",
]
],
orient="v",
palette=adata_cd45.uns["cell_type_lvl5_colors"],
)
ax = sb.swarmplot(
data=df[
[
"Naive CD4+ T cell",
"CD4+ CM T cell",
"CD4+ EM T cell",
"CD4+ TEMRA",
"CD4+ TRM T cell",
"Naive CD8+ T cell",
"CD8+ CM T cell",
"CD8+ EM T cell",
"CD8+ TE T cell",
"CD8+ TRM T cell",
"Double negative T cell",
"Double positive T cell",
]
],
orient="v",
color=".25",
size=5,
alpha=0.8,
)
ax.set_xticklabels(ax.get_xticklabels(), rotation=90)
ax.spines["right"].set_visible(False)
ax.spines["top"].set_visible(False)
ax.set_ylim([-2, 51])
ax.set_ylabel("Percentage of all T cells")
pl.savefig(f"./../figures/{today}_boxplot_Oetjen_cytof.pdf", bbox_inches="tight")
pl.show()
rcParams["figure.figsize"] = (10, 5)
ax = sb.boxplot(
data=df[
[
"Naive CD4+ T cell",
"CD4+ CM T cell",
"CD4+ EM T cell",
"CD4+ TEMRA",
"CD4+ TRM T cell",
"Naive CD8+ T cell",
"CD8+ CM T cell",
"CD8+ EM T cell",
"CD8+ TE T cell",
"CD8+ TRM T cell",
"Double negative T cell",
"Double positive T cell",
]
],
orient="v",
palette=adata_cd45.uns["cell_type_lvl5_colors"],
)
ax = sb.swarmplot(
data=df[
[
"Naive CD4+ T cell",
"CD4+ CM T cell",
"CD4+ EM T cell",
"CD4+ TEMRA",
"CD4+ TRM T cell",
"Naive CD8+ T cell",
"CD8+ CM T cell",
"CD8+ EM T cell",
"CD8+ TE T cell",
"CD8+ TRM T cell",
"Double negative T cell",
"Double positive T cell",
]
],
orient="v",
color=".25",
size=5,
alpha=0.8,
)
ax.set_xticklabels(ax.get_xticklabels(), rotation=90)
ax.spines["right"].set_visible(False)
ax.spines["top"].set_visible(False)
ax.set_ylim([-2, 51])
ax.set_ylabel("Percentage of all T cells")
pl.savefig(f"./../figures/{today}_boxplot_Oetjen_cytof.png", bbox_inches="tight")
pl.show()

End of the annotation notebook.